Quiz-summary
0 of 33 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
Your time:
Time has elapsed
Categories
- Not categorized 0%
- chemistry 0%
- physics 0%
-
Thank you for taking the quiz, you can close the page now.
We will be in touch with you in due course.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- Answered
- Review
-
Question 1 of 33
1. Question
What should be the value of distance d so that final image is formed on the object itself. (Focal lengths of the lenses are written on the lenses):
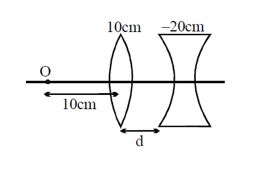
-
Question 2 of 33
2. Question
A plastic bottle contains 0.6 g of N2(g).
Another bottle of the same size is filled at the same temperature and pressure with an unknown gas. If the mass of second gas is 1.52 g, this gas can be :
-
Question 3 of 33
3. Question
From the following data :
C(s) + 2H2(g) → CH4(g) ΔH = – 75kJ/mol
3C(s) + 4H2(g) → C3H8(g) ΔH = – 100kJ/mol
What is ΔH for transformation?
3CH4(g) → C3H8(g) + 2H2(g)
-
Question 4 of 33
4. Question
The following complete reaction is realized:
N2 (g)+ 3 H2 (g) ⟶ 2 NH3 (g)using 0.5 mol of N2 (g) and 2.5 mol of H2 (g).
Under normal conditions of temperature and pressure, what is the volume occupied by the system at the end of the reaction?
-
This response will be reviewed and graded after submission.
-
-
Question 5 of 33
5. Question
Which mass of NaI(s) should you dissolve in 50mL water to obtain a solution of 0.2mol/L in NaI ?
(answer in g)
-
This response will be reviewed and graded after submission.
-
-
Question 6 of 33
6. Question
I’ve got a 2 mol/l aqueous solution of hydrogen chloride. I take 5 mL of it and dilute it with water to a final volume of 100 mL. The final concentration of hydrogen chloride in the resulting solution is:
-
Question 7 of 33
7. Question
What is the mass of 3.2 moles of ammonium nitrate (NH4NO3)? (answer in g)
-
This response will be reviewed and graded after submission.
-
-
Question 8 of 33
8. Question
What is the molar weight of potassium chloride?
-
This response will be reviewed and graded after submission.
-
-
Question 9 of 33
9. Question
The mass of 250mL of liquid benzene is 220g, what is the density if this liquid?
-
Question 10 of 33
10. Question
The charge of a capacitor of 1 μF in a discharging RC circuit decreases by a factor of 2 in 10 milliseconds. Calculate its resistance (write just the number).
-
This response will be reviewed and graded after submission.
-
-
Question 11 of 33
11. Question

-
This response will be reviewed and graded after submission.
-
-
Question 12 of 33
12. Question
The power required to ensure a flow rate Q of water in the following two-pipe system is 0.5
W. (ηwater = 1.10-3 Pas )
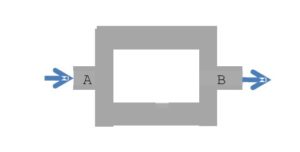
Knowing ΔP= 10Pa between A and B, calculate the flow: (answer in m³/s)
-
This response will be reviewed and graded after submission.
-
-
Question 13 of 33
13. Question
A ball is thrown from the ground with a velocity of 10 m/s following an angle of 30° from the horizontal to a vertical wall, located at 2 m.
How high will it hit the wall? (answer in meter)
-
This response will be reviewed and graded after submission.
-
-
Question 14 of 33
14. Question
Consider the following homogeneous beam of mass m supported by a cable.
Knowing that the beam is in equilibrium, calculate the tension in the cable and the mass m of the beam.
( answer m = ….kg, tension = … N)
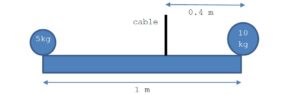
-
This response will be reviewed and graded after submission.
-
-
Question 15 of 33
15. Question
A solution of 0.05M MIBC needs to be prepared from a solution of 100ml of MIBC at 0.1M. How much water should be added in order to obtain this diluted solution?
(answer in mL)
-
This response will be reviewed and graded after submission.
-
-
Question 16 of 33
16. Question
Mark the appropriate geological events associated with the kind of boundaries shown in the following figure.
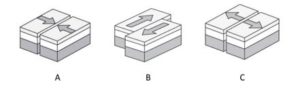
-
Question 17 of 33
17. Question
What is represented in the figure below?
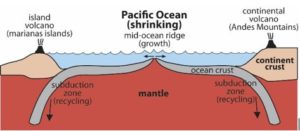
-
Question 18 of 33
18. Question
Which of the following relationships are true?
-
Question 19 of 33
19. Question
“Reporting tonnages of an orebody is a complex task as it directly depends on the delineation of ore boundaries. Any mistakes in identification of boundaries can lead to either misidentification of an orebody or totally ignoring a big part of it. The number of drill holes increases the degree of confidence on the information about the presence of mineralization. Also, overlooking the geological features like fault or folds may lead to discontinuities in the mineralization which might lead to erroneous tonnage estimations”
What could be the possible reasons of uncertainties in identification of a continuous ore body (marked in pink in the figure)?
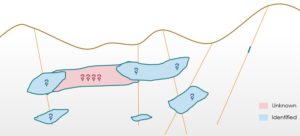
-
Question 20 of 33
20. Question
“Reporting tonnages of an orebody is a complex task as it directly depends on the delineation of ore boundaries. Any mistakes in identification of boundaries can lead to either misidentification of an orebody or totally ignoring a big part of it. The number of drill holes increases the degree of confidence on the information about the presence of mineralization. Also, overlooking the geological features like fault or folds may lead to discontinuities in the mineralization which might lead to erroneous tonnage estimations”
If no mineralization is found in the pink area by additional drilling campaigns. Mark all the possible factors which might be responsible for it?
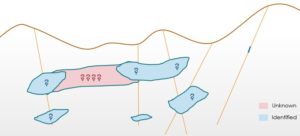
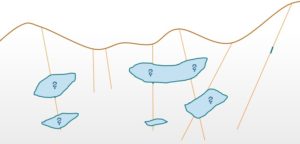
-
Question 21 of 33
21. Question
If 6 underground loaders need 6 minutes to load 6 Mtons of ore on the conveyor system, how many of them are needed to load 80 Mtons of ore in 48 minutes? (write just the number)
-
This response will be reviewed and graded after submission.
-
-
Question 22 of 33
22. Question
Solve this equation:
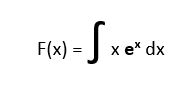
-
Question 23 of 33
23. Question
Solve this equation:
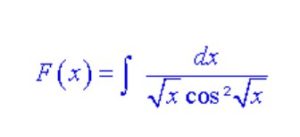
-
Question 24 of 33
24. Question
The keyboard of a machine has 42 keys, including 8 numbers and 26 letters. The keys are typed randomly.
Knowing that the keys are equiprobable, calculate the probability of typing a sequence of 5 letters. (write just the number)
-
This response will be reviewed and graded after submission.
-
-
Question 25 of 33
25. Question
There is a family of 5 children, for each of them, the probability of being a boy or a girl is 1/2.
The probability of having exactly 2 boys and 3 girls in the family is ……. (write just the number)
-
This response will be reviewed and graded after submission.
-
-
Question 26 of 33
26. Question
Consider an Oxy marker in which the coordinates of points B, C and E are B (0, 4), C (3.5, 5), E (5, 0). All position data are in centimeters.
Determine the area of the hexagon defined in the figure below where the angles O, B, C and E are 145°, 150°, 110° and 120° respectively.
(write just the number)
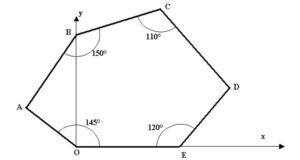
-
This response will be reviewed and graded after submission.
-
-
Question 27 of 33
27. Question
Knowing the following distances, calculate the area of the quadrangle Brussels-Berlin-Athen-Lisboa:
Bruxelles-Berlin : 648km
Bruxelles –Lisbon : 1713km
Bruxelles-Roma : 1182 km
Bruxelles-Athen : 2089km
Lisbon-Berlin : 2310km
Lisbon-Roma :1873km
Berlin-Athen : 1801km
(write just the number of km²)
-
This response will be reviewed and graded after submission.
-
-
Question 28 of 33
28. Question
The temperature in a laboratory rose 6 degrees Celsius, which represents half of the previous temperature. What is the current temperature? (write just the number)
-
This response will be reviewed and graded after submission.
-
-
Question 29 of 33
29. Question
“A rhyolitic tuff from Nevada (USA) containing 1 g / ton is valuable today given the price of gold and average operating costs in large scale mining operations”.

According to today’s gold price (41.21 €/g) what should be the maximum limit of production cost/ton of ore to maintain the gold grade profitable?
-
Question 30 of 33
30. Question
“A rhyolitic tuff from Nevada (USA) containing 1 g / ton is valuable today given the price of gold and average operating costs in large scale mining operations”.

Which of the following inferences cannot be drawn from the statement presented above?
-
Question 31 of 33
31. Question
In an iron ore mine, one of the processing plants is currently fed with 200t/h of ore to produce 160t/h of hematite concentrate at 68% Fe grade. A study to improve the plant’s efficiency showed that the increase of 2 flotation cells in the rougher stage of flotation could improve the plant recovery in 4% with a small decrease of 1% in Fe grade. If the market currently pays 120€ per ton of Fe in the concentrate, what would be the hourly increase in revenue obtained by installing these 2 cells? Note: do not consider any additional operational or installation costs.
(answer in EUR/h)
-
This response will be reviewed and graded after submission.
-
-
Question 32 of 33
32. Question
Sedimentary rocks could form upon weathering, erosion, sedimentation and lithification of_________
-
Question 33 of 33
33. Question
A deposit with a grade of 2.5% Zn and with an ore body of density 2.5 g.cm3, will be mined by open pit method. The dimension of the open pit will be 200 m depth, 100 m width and 300 m length.
How many tonnes of Zinc can be extracted? (write just the number)
-
This response will be reviewed and graded after submission.
-